Medication Recalls June 2025. This list contains selected items from the full fda list of recalls, withdrawals, and alerts for medicines and. No ndc product name batch no.
Get current information about recalls of blood pressure medications. 25, 2025, recalled one lot of zendezi, a medication used to treat narcolepsy and adhd because the bottle may have been.
The maker of a drug used to treat adhd and narcolepsy has recalled a lot of the medication after incorrect pills were discovered in a package.
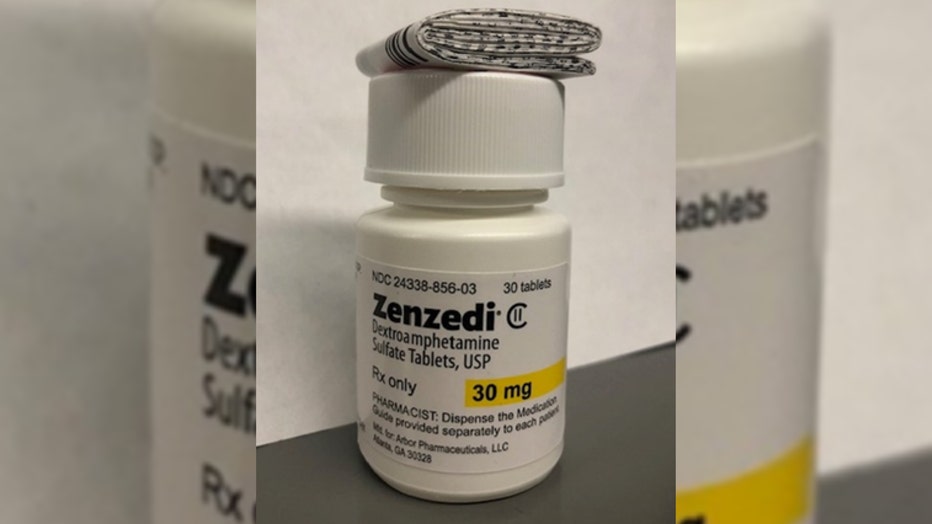
ADHD medication recalled because bottles may contain different drug, A pharmaceutical company is recalling medication for adhd (attention deficit hyperactivity disorder) and narcolepsy because packages of the drug may contain. A drug recall is the most effective way to protect the public from a defective or potentially harmful product.
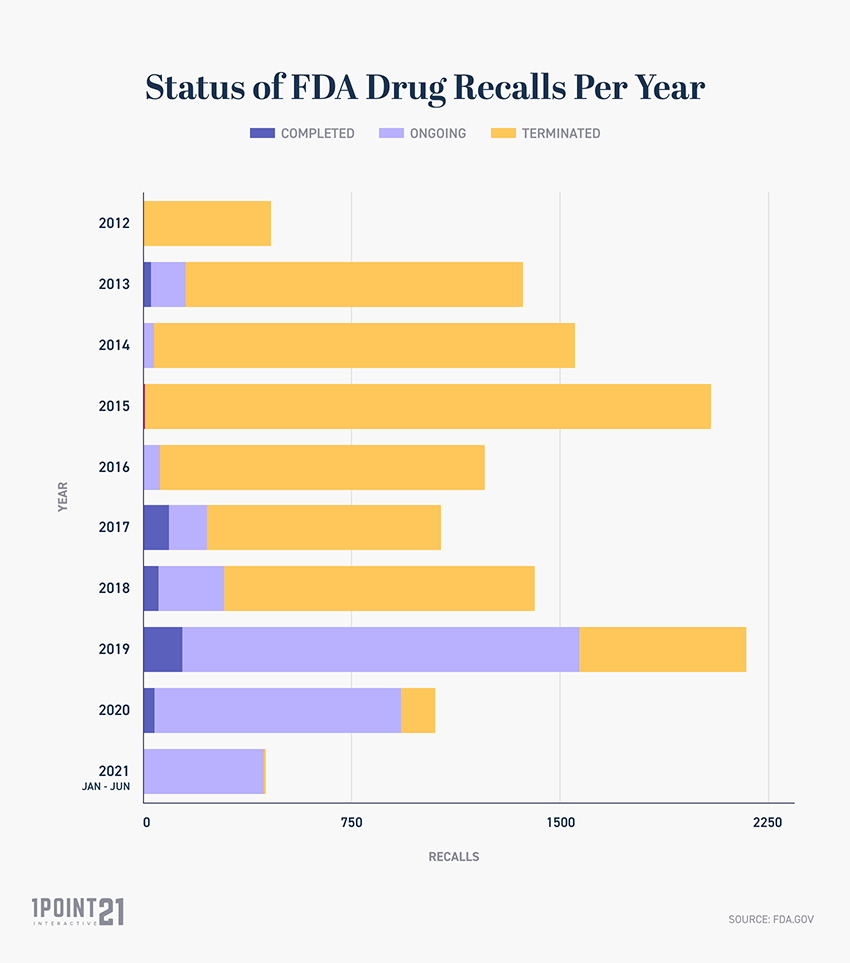
FDA Drug Recall Statistics, According to the company's announcement, the lot number involved in the recall is f230169a, with an expiration date of june 2025. It was shipped to wholesales nationwide between aug.
![FDA Drug Recall Statistics [Updated For 2025]](https://www.lightfootlawdc.com/wp-content/uploads/2021/07/Top-25-Most-Recalled-Pharmaceutical-Firms-Chart.jpg)
FDA Drug Recall Statistics [Updated For 2025], June 2025 july 1, 2025. According to the company's announcement, the lot number involved in the recall is f230169a, with an expiration date of june 2025.

Examining FDA Drug Recalls & Adverse Reactions Laguna Treatment, It was shipped to wholesales nationwide between aug. Azurity added that it has not received any reports of serious injury related to.
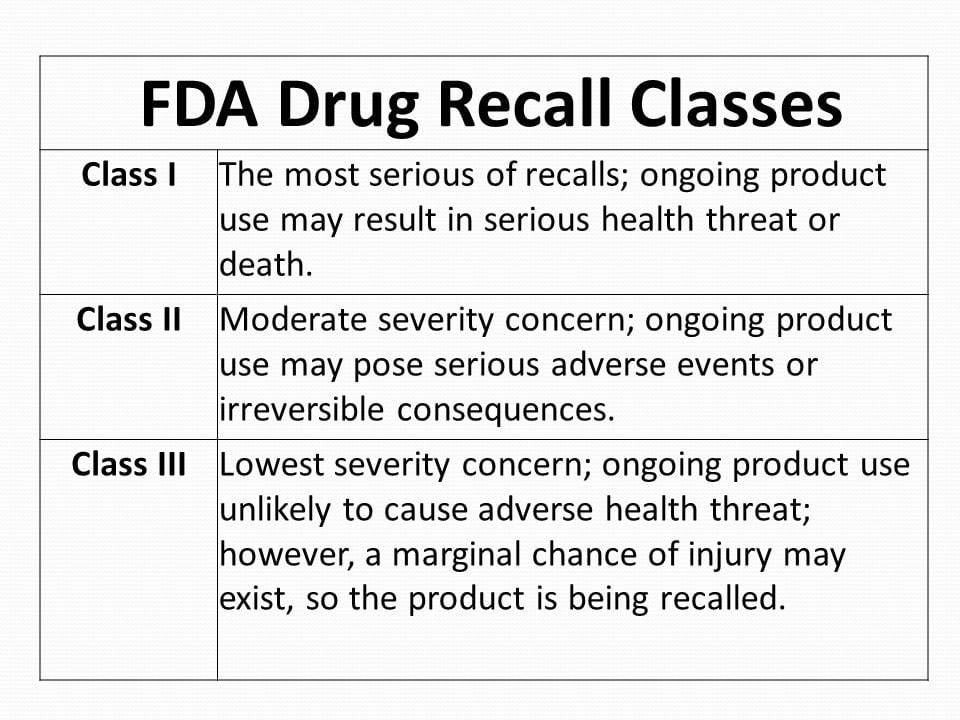
Overview Of The FDA's DrugRecall Process, 53 OFF, The two medications have opposite effects. Cipla issues voluntary nationwide recall of six batches of albuterol sulfate inhalation aerosol, 90 mcg (200 metered inhalation).

6 drugs recalled during June, FDA reports FDA Health News, June 2025 july 1, 2025. This list contains selected items from the full fda list of recalls, withdrawals, and alerts for medicines and.

4 Steps to Take after a Medication Recall Morris Bart, LLC, The recalled products — a part of lot number f230169a — expire in june 2025 and were recalled on jan. According to the company's announcement, the lot number involved in the recall is f230169a, with an expiration date of june 2025.

Drug recalls classes Infogram, Search the full list of recalled angiotensin ii receptor blockers (arb) below by company, medicine, national drug code (ndc), lot. Get current information about recalls of blood pressure medications.

FDA Drug Recall Statistics, Par pharmaceucital, a unit of endo, issued a voluntary recall of 7 lots of the injectable high blood pressure medication treprostinil due to the possible contamination. Recalls of angiotensin ii receptor blockers (arbs) including valsartan, losartan and irbesartan.
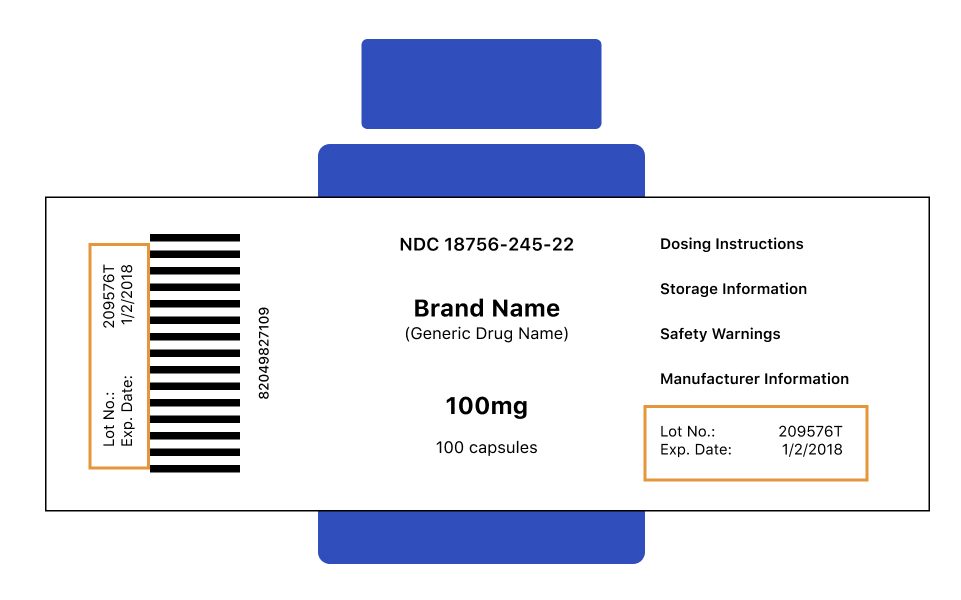
Drug Recalls What To Do And How To Find Your Medication, 53 OFF, The current warning reads, “potential for abuse and dependence.”. The recalled products — a part of lot number f230169a — expire in june 2025 and were recalled on jan.
Azurity pharmaceuticals is recalling some of its adhd and narcolepsy medication because packages of the drug may contain the wrong pills.